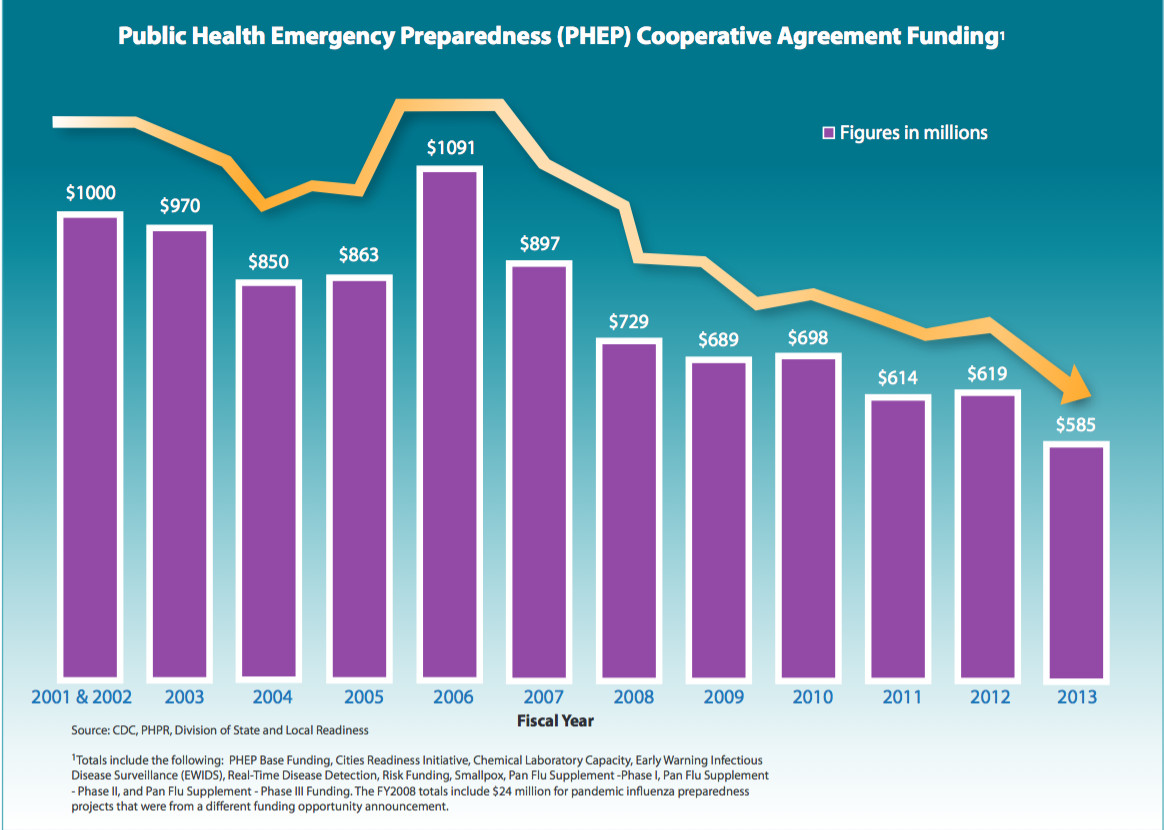
The impact of funding cuts on medical research has emerged as a significant concern for the future of healthcare and scientific advancement. In recent months, dramatic reductions in medical research funding, including a notable freeze of over $2 billion by the federal government, jeopardize patient safety in research and undermine ethical research practices. Without adequate resources, the delicate balance that institutional review boards (IRBs) maintain in overseeing research studies is at risk of collapsing. Leading institutions, such as Harvard, which rely on grants to propel innovative projects forward, are now facing disruptions that could stall groundbreaking discoveries. As we explore this crisis, it becomes clear that ensuring the safety and well-being of research participants hinges on the consistent availability of support for these essential research initiatives.
The repercussions of budgetary constraints on scientific exploration represent a critical challenge for today’s research landscape. Severe reductions in medical research financing threaten to compromise patient well-being and hinder the ethical execution of clinical studies. As traditional funding bodies withdraw their financial support, prestigious research institutions, including Harvard, are left scrambling to accommodate studies that require rigorous oversight and compliance. The halt in funding not only stymies research progress but also risks the ethical integrity that underpins collaborative projects reviewed by institutional review boards (IRBs). In this era of uncertainty, the importance of sustaining research financing cannot be overstated, as it safeguards both the innovation process and the rights of individuals who participate in life-saving medical trials.
The Importance of Medical Research Funding
Funding for medical research is critically important as it lays the foundation for innovative discoveries in healthcare. Federal grants, such as those from the National Institutes of Health (NIH), provide essential financial resources that enable researchers to investigate new treatments, develop medical technologies, and advance our understanding of diseases. Without adequate funding, many promising research initiatives may never move beyond the planning stages, leading to a significant setback in medical advancements that could potentially benefit countless patients.
Additionally, the intricate nature of medical research often requires collaboration across various sites and institutions. The availability of funding ensures that these collaborative efforts can be effectively managed. It allows for the establishment of systems such as the SMART IRB, which streamlines oversight by facilitating the review of research protocols across multiple locations. When funding cuts occur, the integrity and continuity of these collaborative efforts are jeopardized, ultimately hindering the progress of vital research.
Impact of Funding Cuts on Medical Research
The cessation of funding can have devastating effects on ongoing medical research projects. When federal support is cut, institutions are compelled to halt or scale back research efforts abruptly. This not only disrupts the work being conducted but also puts a strain on the relationships built with study participants, many of whom have invested their time and trust in the research process. As funding cuts force institutions like Harvard to pause studies, more than 25 additional sites are also barred from participating, stalling significant advancements in potential therapies.
Moreover, the impact of funding cuts can extend beyond immediate delays. When researchers are unable to proceed with their studies, it contributes to a growing skepticism among the public regarding the intentions and capabilities of the medical research community. The historical breaches of trust in medical research emphasize the importance of transparent and ethical oversight, which can be compromised when financial challenges arise. This erosion of trust can deter potential participants, fundamentally altering the landscape of clinical research and diminishing the quality of data gathered.
Role of IRBs in Patient Safety
Institutional Review Boards (IRBs) serve as the backbone of ethical oversight in medical research, ensuring that patient safety is prioritized. The IRB’s primary function is to review research proposals meticulously, assessing not only the scientific merit of the studies but also the ethical implications involved. These boards are responsible for safeguarding the rights and well-being of research participants by enforcing policies that mandate informed consent, monitor adverse events, and mitigate risks associated with research participation.
Furthermore, IRBs are pivotal in creating a system of checks and balances that govern ethical research practices. They offer training and resources to research teams, ensuring that all aspects of the study align with established ethical guidelines. As champions of patient safety, IRBs foster an environment where participants understand their roles in research and feel confident about their involvement. A well-functioning IRB can facilitate a much smoother research process, enhancing collaboration among institutions and streamlining operations.
Ethics in Medical Research: A Historical Perspective
Understanding the historical context of medical research ethics is crucial in framing contemporary practices. Past injustices, such as the Tuskegee Study and the Willowbrook hepatitis studies, highlighted the hypocrisy of conducting research without proper consent and oversight. These incidents led to the establishment of stricter regulations governing human subjects research, including the creation of IRBs, which exist to prevent ethical breaches and safeguard participant rights. The evolution of these ethics reflects society’s commitment to ensuring that all research is conducted transparently and with respect for individual autonomy.
Today, researchers are held to high ethical standards that require them to navigate complex regulatory landscapes. Modern frameworks, such as the Belmont Report, emphasize respect for persons, beneficence, and justice, guiding researchers in their ethical obligations. The commitment to ethics is not only a regulatory requirement but also a moral imperative that underscores the trust necessary to foster public participation in medical research. Ethical scrutiny remains vital as new technologies and methodologies emerge, ensuring that the potential benefits of research do not overshadow the rights of individuals.
Future of Medical Research Amid Funding Challenges
The future of medical research faces uncertainty, primarily due to increasing funding challenges that threaten the sustainability of projects essential for public health. As institutions navigate budget cuts and dwindling grants, researchers are forced to critically evaluate which studies can be continued or initiated. This decision-making process can lead to a narrow focus on projects that align strictly with available funding, potentially leaving important areas of inquiry unexplored.
Moreover, the reliance on grants from institutions like Harvard often restates the pressing need for diversified funding sources. To maintain robust and innovative research programs, institutions must seek partnerships with private foundations, industry sponsors, and international research collaborations. Effective communication about the value and impact of research is paramount in securing these alternative funding avenues. The collective efforts of the research community, advocacy groups, and policymakers will be crucial in reshaping the landscape of scientific inquiry, ensuring continued progress in the face of fiscal challenges.
Navigating Ethical Research Practices
Ethical research practices are foundational to the integrity of scientific inquiry. Researchers are required to adhere to established guidelines that govern the treatment of human subjects, encompassing everything from informed consent to the management of confidentiality. By following these practices, researchers not only protect participants but also uphold the credibility of the research community. Ethical violations can lead to severe repercussions, including legal consequences and a loss of public trust, underscoring the necessity of maintaining high ethical standards.
Institutions must cultivate an environment that prioritizes ethical conduct in research through continuous training and education. Researchers should be encouraged to remain aware of ethical considerations at every stage of their work, ensuring that the safety and welfare of participants remain paramount. Furthermore, fostering an open dialogue about ethical dilemmas faced in research can empower teams to seek guidance and make decisions that align with moral and regulatory expectations. By integrating ethical considerations into the fabric of research culture, institutions can enhance their capacity to conduct responsible and impactful studies.
The Role of Technological Innovations in Research Oversight
Technological innovations are transforming the landscape of medical research oversight, enhancing the effectiveness and efficiency of IRBs and compliance processes. Electronic platforms for submitting research protocols have streamlined application procedures, allowing for faster review times and improved accessibility for researchers across multiple sites. These technological advances also facilitate better communication among stakeholders, enabling real-time updates on the status of research studies and compliance with regulatory standards.
Moreover, data management systems can significantly bolster participant safety by ensuring that adverse events are thoroughly tracked and reported. By deploying advanced analytics, IRBs can monitor studies more effectively and identify potential ethical concerns before they escalate. Embracing technology bolsters the research community’s commitment to safeguarding participants and supporting ethical research practices in an increasingly complex environment. As reliance on technology grows, continuous adaptation and training will be vital to maximize its benefits and effectiveness.
Public Engagement and Trust in Medical Research
The relationship between medical research institutions and the public is foundational to the success of research initiatives. Gaining public trust is crucial, particularly as funding cuts and ethical concerns can lead to skepticism about the motives and integrity of research. Engaging with communities through outreach programs, educational initiatives, and transparency in the research process can help rebuild trust and foster a collaborative spirit between researchers and participants.
Public engagement efforts should prioritize inclusive dialogues that address community concerns and emphasize the benefits of participation in research. By actively involving community stakeholders in the design and execution of studies, researchers can tailor their approaches to better meet the needs of the populations they serve. Increased transparency regarding research goals, methods, and outcomes further empowers individuals to make informed decisions about their participation. Ultimately, building trust and maintaining open lines of communication between the research community and the public are essential for the continued progress of medical research.
Strategies for Securing Sustainable Medical Research Funding
In the face of funding challenges, it is crucial for research institutions to adopt innovative strategies for securing sustainable financial support. Diversifying funding sources can mitigate the risks associated with reliance on federal grants alone. This may include establishing partnerships with private organizations, philanthropic foundations, and healthcare institutions that recognize the value of advancing medical research. By demonstrating the potential impact of their work, researchers can attract investments that align with both community health needs and institutional goals.
Another effective strategy involves fostering collaborations with industry partners to leverage additional resources and expertise. These partnerships can not only enhance research capacity but also ensure that studies are aligned with real-world applications. Engaging stakeholders in the research process can foster a sense of shared investment in outcomes, motivating sustained support. By implementing these strategies, institutions can create a resilient funding ecosystem that facilitates ongoing research efforts and contributes to the advancement of science and healthcare.
Frequently Asked Questions
What is the impact of funding cuts on medical research funding and patient safety?
Funding cuts to medical research funding can have profound adverse effects on patient safety. Reductions in funding lead to fewer resources for necessary Institutional Review Board (IRB) oversight, which is critical for ensuring that research studies adhere to ethical practices and prioritize participant safety. Without adequate funding, IRBs may struggle to perform comprehensive reviews of research protocols, potentially increasing risks for participants involved in these studies.
How do cuts in medical research funding affect the role of IRB oversight?
Cuts in medical research funding significantly hamper the operational capacity of IRBs, which are essential for overseeing research ethics and patient safety. Insufficient funding can limit the ability of IRBs to conduct thorough reviews and provide necessary training for researchers, ultimately jeopardizing the integrity of clinical research and the rights of study participants.
What happens to medical research when funding cuts occur?
When funding cuts occur, numerous medical research projects may face immediate freezes or cancellations, disrupting ongoing studies and delaying the introduction of new therapies. This halt can prevent research institutions from expanding their collaborative efforts, thereby adversely impacting advancements in medical knowledge and treatment options for patients.
How do Harvard research grants support ethical research practices amidst funding cuts?
Harvard research grants play a crucial role in supporting ethical research practices, especially during times of funding cuts. These grants enable researchers and their affiliated IRBs to maintain oversight and governance, ensuring that ethical standards are upheld even when federal funding is compromised. They provide a buffer, allowing essential studies to continue without sacrificing participant safety.
What are the long-term consequences of funding cuts on medical research and patient safety?
The long-term consequences of funding cuts on medical research can be detrimental. They may lead to a decline in the number of clinical trials, reduced public trust in medical research, and ultimately slower progress in discovering new treatments. As funding diminishes, the capacity for ethical oversight by IRBs can also decline, further threatening the safety of participants in research studies.
How does funding impact the collaboration of institutions in medical research?
Funding is vital for fostering collaboration among institutions in medical research. When funding is cut, there are barriers to cooperative efforts, such as multisite trials, which can stall research needed for critical health advancements. Institutions may be less inclined to engage in partnerships without the assurance of adequate financial support, hampering progress in addressing urgent health issues.
What role does the federal government play in medical research funding and ethical oversight?
The federal government plays a significant role in medical research funding by providing grants and establishing policies that promote ethical oversight through IRBs. Federal funds help ensure that research is conducted ethically, safeguarding the rights and welfare of participants. When these funds are cut, the structural support for both research and ethical oversight is weakened, impacting the overall integrity of medical research.
Why is it important to maintain funding for IRB oversight in medical research?
Maintaining funding for IRB oversight is essential to protect the rights and safety of individuals participating in medical research. Adequate funding ensures that IRBs can perform their critical functions, such as reviewing research proposals and monitoring compliance with ethical standards. This oversight is vital for preserving public trust in medical research and preventing potential harm to participants.
| Key Aspect | Details |
|---|---|
| Impact of Funding Cuts on Medical Research | Funding cuts disrupt oversight systems like SMART IRB, which are crucial for ensuring patient safety in multi-site medical research. |
| SMART IRB’s Function | SMART IRB streamlines the review process for collaborative research across various institutions, safeguarding participant rights. |
| Role of Institutional Review Boards (IRBs) | IRBs ensure ethical research practices, informed consent, and participant safety, serving as an essential oversight mechanism. |
| Historical Context of IRBs | Established in response to unethical research practices, IRBs protect participants based on historical learnings from past medical abuses. |
| Consequences of Halted Research | Cuts result in delayed studies, reduced participant recruitment, and growing skepticism towards medical research. |
| Importance of Continued Funding | Maintaining funding is vital for the ongoing protection of participants and for advancing medical research innovations. |
Summary
The impact of funding cuts on medical research is profound, affecting the safety and rights of participants involved in crucial studies. With significant recent funding losses, particularly at institutions like Harvard, essential oversight systems such as the SMART IRB face disruptions that jeopardize patient protection measures. Historical precedents demonstrate the necessity for rigorous ethical standards in medical research; thus, the cancellation of grants hampers efforts to uphold these commitments. Continued funding is essential not only for the safeguarding of research participants but also for the broader integrity and progress of scientific inquiry.