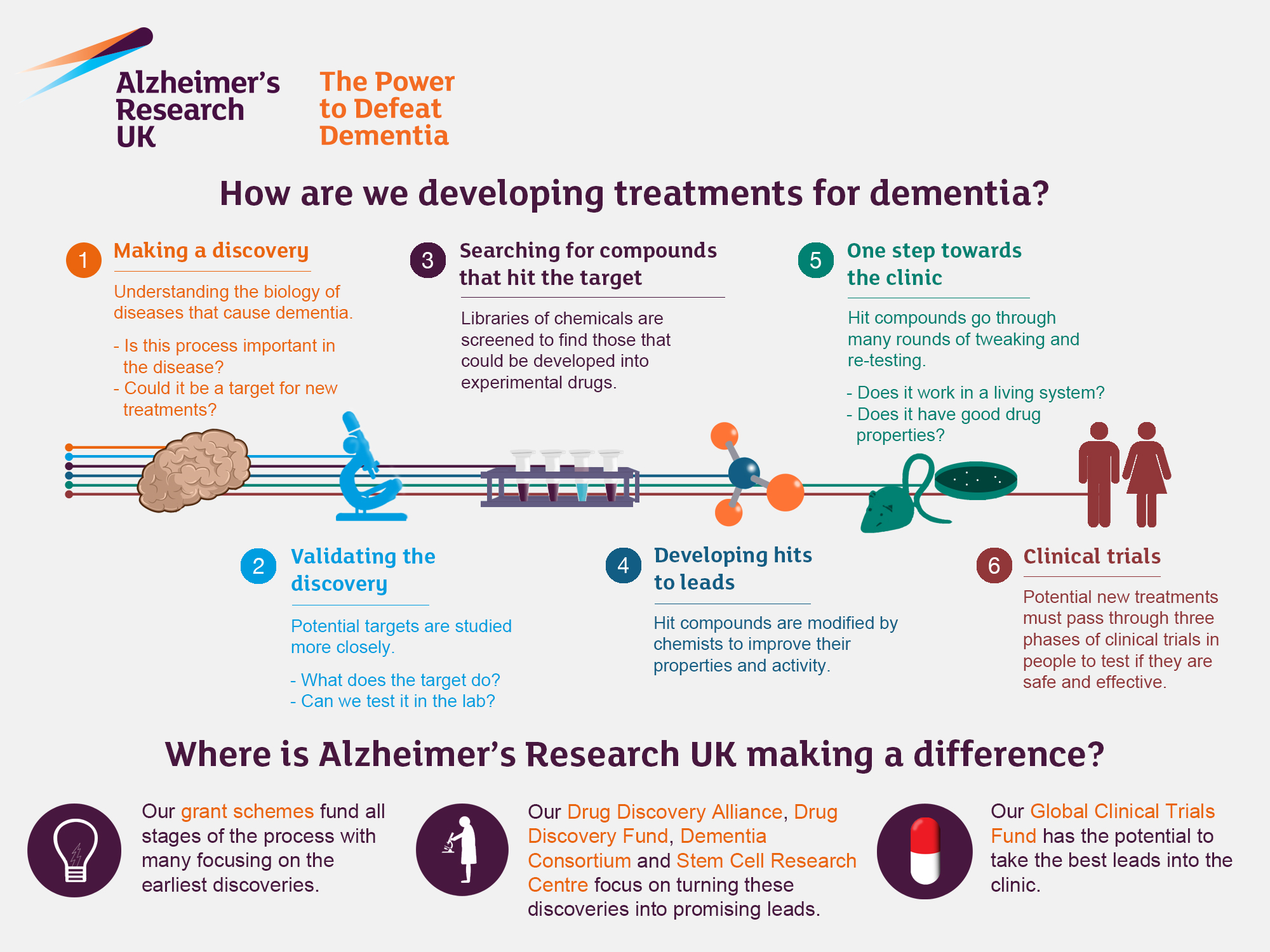
Alzheimer’s research is at the forefront of neuroscience, aiming to unravel the complexities of this devastating disease that affects millions worldwide. Leading the charge is Beth Stevens, a pioneering neuroscientist whose work focuses on microglial cells, the brain’s immune system that plays a critical role in maintaining neuronal health. As she investigates the process of synaptic pruning—where microglia remove dead cells and damaged neurons—Stevens has uncovered links to Alzheimer’s disease, suggesting that improper pruning could exacerbate neurodegenerative diseases. Her groundbreaking findings not only illuminate potential pathways for effective Alzheimer’s treatment but also pave the way for identifying earlier biomarkers, which are crucial for timely interventions. With the number of Alzheimer’s cases projected to double by 2050, understanding and enhancing our brain immune system’s function is more vital than ever.
Exploring the domain of Alzheimer’s research reveals an intricate web of scientific inquiries dedicated to combating one of humanity’s most pressing health challenges. The work of experts like Beth Stevens highlights the pivotal role of brain immune cells, known as microglia, which actively manage the delicate balance of brain health by clearing out cellular debris. This research not only addresses the broader landscape of neurodegenerative disorders but also sheds light on the nuances of synaptic maintenance, offering fresh perspectives on potential therapies for Alzheimer’s disease. As we delve into this essential field, the insight into how our brain defends itself opens new avenues for treatment, contributing to the hope for better care strategies for those impacted by these conditions.
Understanding Microglial Cells in Alzheimer’s Research
Microglial cells are the brain’s innate immune cells, essential for maintaining neural health. In the context of Alzheimer’s research, these cells play a pivotal role in how the brain handles cellular debris and inflammation. When functioning correctly, microglia can prune unnecessary synapses during brain development and repair damaged neurons. However, abnormal microglial activity has been linked to the progression of neurodegenerative diseases like Alzheimer’s, where their failure to efficiently clear toxic proteins leads to plaque formation and neuronal death.
Beth Stevens’ groundbreaking work has illuminated the dual role of microglial cells in both protecting and damaging the brain. Her research has revealed that while microglia are essential for normal brain function, their overactive response during neurodegenerative conditions can exacerbate neuronal degeneration. This understanding opens avenues for targeted Alzheimer’s treatments that could modulate microglial activity, potentially mitigating their harmful effects while enhancing their protective functions.
The Link Between Neurodegenerative Diseases and Microglial Pruning
Neurodegenerative diseases, including Alzheimer’s and Huntington’s disease, have been found to share a common pathological feature: abnormal microglial pruning of synapses. This improper refinement of neuronal connections disrupts the delicate balance of synaptic networks in the brain, leading to cognitive decline. Stevens’ research has established that inadequate or excessive pruning by microglia contributes to neuronal loss, highlighting the importance of these immune cells in maintaining brain health.
Through her pioneering studies, Stevens has demonstrated that targeted interventions could potentially rectify the malfunctioning role of microglia in Alzheimer’s disease. By understanding how microglial cells interact with synapses and contribute to disease progression, researchers can develop therapeutic approaches that not only prevent synapse loss but also restore cognitive function. This could transform how we approach treatment and prevention strategies for a growing population at risk of neurodegenerative diseases.
Beth Stevens: Pioneering the Future of Alzheimer’s Treatment
Beth Stevens’ work exemplifies the intersection of curiosity-driven science and practical application in Alzheimer’s treatment. By delving into the behaviors and functions of microglial cells, her lab has uncovered critical insights that could enhance our understanding of Alzheimer’s. These findings are monumental, paving the way for the development of new biomarkers that allow for earlier diagnosis and intervention, which is essential as cases of Alzheimer’s continue to rise due to aging populations.
In Stevens’ view, the support from federal funding agencies has been vital in advancing Alzheimer’s research. Her projects are not just theoretical; they have the potential to translate into real-world benefits, helping to change the prognosis for millions of Americans affected by Alzheimer’s and related neurodegenerative conditions. As the landscape of Alzheimer’s treatment evolves, her insights into the brain’s immune system could soon lead to breakthroughs that shift the paradigm of care and improve the quality of life for patients.
The Role of the Brain Immune System in Alzheimer’s Disease
The brain immune system, primarily mediated by microglial cells, plays a crucial role in Alzheimer’s disease progression. In healthy brains, microglia perform necessary functions, including removing cellular debris and supporting neuronal growth. However, in Alzheimer’s, the malfunction of microglial cells can lead to an inflammatory response that exacerbates the disease. By understanding this immune response, researchers hope to find ways to harness or regulate microglial activity, potentially transforming the approach to Alzheimer’s treatment.
Insights from scientists like Beth Stevens have shown that manipulating microglial behavior could offer new strategies for intervention. By reducing harmful inflammation or enhancing the protective roles of microglia, new therapies could emerge that not only address symptoms but also target the underlying causes of Alzheimer’s. The brain’s immune system presents a promising frontier for innovative treatments that aim to halt or even reverse the neurodegenerative processes characteristic of Alzheimer’s disease.
Early Detection Through Biomarkers in Alzheimer’s Research
Developing effective biomarkers for early detection of Alzheimer’s is critical for improving patient outcomes. Innovations stemming from Beth Stevens’ research on microglial cells have the potential to identify biochemical changes in the brain that occur long before cognitive symptoms manifest. This early detection could enable preventative strategies and more effective Alzheimer’s treatment methodologies, significantly impacting the lives of millions at risk.
The establishment of microglial cell behavior as a biomarker offers a window into the Alzheimer’s disease process. As research continues, these biomarkers could lead to routine screenings that detect Alzheimer’s in its asymptomatic stages, paving the way for early therapeutic interventions. This paradigm shift in how we approach Alzheimer’s—focusing on prevention and early treatment—could fundamentally change the prognosis for those at risk and help tackle the burgeoning Alzheimer’s epidemic.
Funding and Support for Alzheimer’s Research
Federal funding has played an indispensable role in propelling Alzheimer’s research forward. Scientists like Beth Stevens have relied heavily on grants from institutions like the National Institutes of Health (NIH) to explore the complexities of neurodegenerative diseases. As research unveils the mechanisms behind conditions like Alzheimer’s, continued financial support is crucial for translating these discoveries into effective treatments and interventions.
With a staggering increase in the number of Alzheimer’s cases projected, securing funding is more vital than ever. It enables researchers to investigate innovative ideas that could lead to breakthrough therapies, enhancing understanding of the neuroimmune system and its implications in Alzheimer’s treatment. As the need for solutions grows, so does the urgency for sustained investment in scientific exploration dedicated to combating this devastating disease.
Impact of Aging on Alzheimer’s Disease Prevalence
As the global population ages, the prevalence of Alzheimer’s disease is anticipated to escalate dramatically. Experts predict that the number of Americans living with Alzheimer’s could double by 2050, emphasizing the urgent need for ongoing research efforts. This rising tide of cases not only poses a challenge for healthcare systems but also underscores the importance of understanding the biological underpinnings of Alzheimer’s, particularly the role of microglial cells.
The aging process is closely linked to the inflammatory response seen in Alzheimer’s disease. Research has shown that older individuals experience heightened microglial activation, which, while protective in youth, can become detrimental with age, contributing to neurodegeneration. Understanding how age-related changes in the brain’s immune system influence Alzheimer’s disease is essential for developing targeted interventions aimed at mitigating its impact as our populations continue to age.
Integrative Approaches to Alzheimer’s Treatment
The fight against Alzheimer’s disease requires multidisciplinary collaboration and integrative approaches. By pooling knowledge from neurobiology, pharmacology, and immunology, researchers can develop comprehensive strategies that address the multifaceted aspects of Alzheimer’s. The pioneering work by scientists like Beth Stevens demonstrates how insights into microglial cells can inform drug development and treatment methodologies, combining to enhance patient care in the arena of neurodegenerative diseases.
By leveraging the complexities of the brain’s immune response, integrative treatment programs can be formulated to target not just the symptoms but the underlying disease mechanisms. Ongoing research will continue to reveal how understanding microglial behavior can inform holistic approaches that encompass lifestyle changes, pharmacological options, and innovative therapies, ultimately aiming to improve the quality of life for those affected by Alzheimer’s disease.
Future Directions in Alzheimer’s Research
The future of Alzheimer’s research is bright, with continued innovations and discoveries on the horizon. Beth Stevens’ insights into microglial cells not only deepen our understanding of the disease but also shape new research questions and therapeutic targets. Moving forward, scientists will focus on elucidating the intricate interactions between microglia, neurons, and other cell types in the brain to develop nuanced strategies for intervention.
Moreover, as we strive toward more personalized medicine approaches, understanding individual variability in immune response and neuroinflammation will be crucial. Future clinical trials will likely test new therapies that specifically modulate microglial activity, aiming to enhance cognitive function and slow disease progression. With ongoing investment and dedication, the aim is to make substantial strides in combating Alzheimer’s disease, ultimately changing the lives of millions impacted by this condition.
Frequently Asked Questions
What role do microglial cells play in Alzheimer’s research?
Microglial cells are crucial in Alzheimer’s research as they function as the brain’s immune system, patrolling for illness and injury. They help clear out damaged cells and prune synapses. However, in Alzheimer’s disease, aberrant pruning by microglia can contribute to neurodegenerative processes. Understanding their function is vital for developing new treatments for Alzheimer’s and other neurodegenerative diseases.
How does Beth Stevens contribute to Alzheimer’s research?
Beth Stevens, a prominent neuroscientist, has significantly advanced Alzheimer’s research by studying microglial cells and their role in the brain’s immune system. Her work has uncovered how incorrect pruning by these cells can lead to Alzheimer’s and other neurodegenerative diseases, paving the way for new therapies and early detection biomarkers for Alzheimer’s.
What are the implications of microglial research for Alzheimer’s treatment?
Research on microglial cells has profound implications for Alzheimer’s treatment. By understanding how these immune cells interact with neurons and contribute to diseases like Alzheimer’s, researchers can create targeted therapies that improve or restore the brain’s immune response, potentially slowing the progression of neurodegenerative diseases.
Why is transforming our understanding of the brain’s immune system important for Alzheimer’s research?
Transforming our understanding of the brain’s immune system, especially through the study of microglial cells, is essential for Alzheimer’s research because it highlights how immune responses can affect neuronal health. This knowledge is critical for developing effective treatments and interventions that address the underlying mechanisms of Alzheimer’s and enhance brain health.
How can studying microglial cells lead to earlier detection of Alzheimer’s disease?
Studying microglial cells can lead to the identification of biomarkers indicative of Alzheimer’s disease. By understanding how microglial activity changes in response to neurodegenerative processes, researchers can develop early detection methods that may allow for timely interventions and improved patient outcomes.
What challenges does Alzheimer’s research face in light of increasing cases?
Alzheimer’s research faces significant challenges as the number of diagnosed cases is expected to double by 2050. These challenges include the need for increased funding, the complexity of neurodegenerative diseases, and the urgent demand for effective treatments as the aging population grows, which could strain healthcare resources.
What is the potential impact of Beth Stevens’ Alzheimer’s research on future therapies?
Beth Stevens’ Alzheimer’s research has the potential to revolutionize future therapies by establishing new approaches based on the function of microglial cells. Her work can lead to innovative treatment options and enhance strategies to address neurodegenerative diseases, positively impacting the quality of life for millions affected by Alzheimer’s.
| Key Points | Details |
|---|---|
| Neuroscientist Role | Beth Stevens, associate professor at Harvard Medical School, focuses on microglial cells and their role in brain health. |
| Microglial Cells | These cells act as the brain’s immune system, clearing out dead cells and pruning synapses, which can affect neurodegenerative diseases. |
| Research Impact | Stevens’ research shows that abnormal pruning is linked to Alzheimer’s and other neurodegenerative disorders, shaping future treatments. |
| Public Health Concern | The number of Alzheimer’s cases in the U.S. is expected to rise significantly, from 7 million now to 14 million by 2050. |
| Funding Importance | Stevens’ work has been largely supported by federal funding, illustrating the need for investment in basic science. |
| Future of Treatments | With new discoveries in microglial research, effective treatments and early detection methods for Alzheimer’s could emerge. |
Summary
Alzheimer’s research is crucial in uncovering the underlying mechanisms of the disease as scientists like Beth Stevens explore the role of microglial cells in brain function. Her groundbreaking work highlights how understanding the immune system of the brain can lead to innovative treatments and strategies for early detection. As we anticipate a significant rise in Alzheimer’s cases, such research becomes indispensable in developing solutions to combat this growing health crisis.